Overview
The Tanner lab applies the tools of organic chemistry to answer problems in biological chemistry. Questions of interest involve enzyme mechanism, inhibitor design, and biosynthesis. Organic synthesis is employed to prepare isotopically labeled substrates, alternate substrates, and enzyme inhibitors. The concepts of physical organic chemistry are used in the analysis of reaction mechanisms and in the rational design of mechanism-based inhibitors. Biochemical techniques such as protein overproduction, protein purification, kinetic analysis of enzymatic reactions, and site-directed mutagenesis are all performed in house. Collaborations with X-ray crystallographers and microbiologists expand the toolkit available to tackle a given problem.
Current Research Projects
Cell Shape-Determining Enzymes (Csd’s): Pathogenic bacteria such as Helicobacter pylori (the cause of ulcers and gastric cancer) and Campylobacter jejuni (a major cause of food poisoning) have a helical shape that is required for infectivity. A series of proteases called Csd’s have recently been discovered that are responsible for the maintenance of the cell shape in these organisms. Knocking out these enzymes results in a straight rod phenotype and reduced pathogenicity. The metalloprotease Csd4 hydrolyzes the bond between iso-D-Glu and meso-Dap in uncrosslinked strands of peptidoglycan (PG). Similarly, the cysteine protease Csd6 hydrolyzes the bond between meso-Dap and D-Ala in uncrosslinked strands of peptidoglycan. Research in the Tanner lab involves the synthesis of peptide substrates, the kinetic analysis of these reactions, and the design of inhibitors of these enzymes.
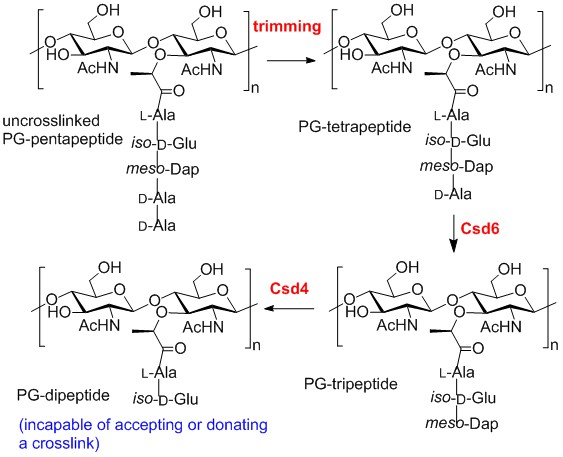
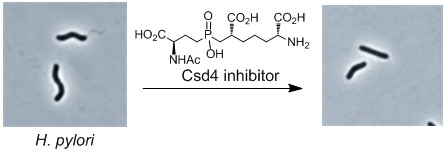
- C. K. Chan et al., J. Biol. Chem. 2015, 290, 3622-3628. Y. Liu et al., ACS Chem. Biol. 2016, 11, 981-991.
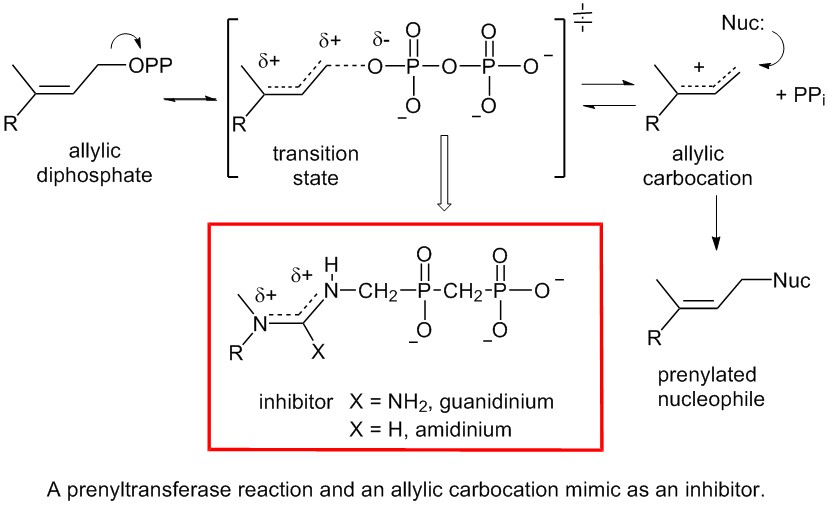
Prenyltransferase inhibitors: Prenyltransferases are enzymes that catalyze the transfer of prenyl groups onto a variety of nucleophiles using allylic diphosphates. Inhibitors of prenyltransferases could be useful as anti cancer agents (farnesyl diphosphate synthase, protein farnesyltransferase), antiparasitic agents (farnesyl diphosphate synthase), and cholesterol lowering agents (squalene synthase). All prenyltransferases catalyze reactions by the formation of an allylic carbocation (or a TS with a great deal of carbocation character). Research in the Tanner lab is focusing on the synthesis and testing of inhibitors that utilize a guanidinium or amidinium moiety to mimic the planer delocalized allylic carbocation intermediate. These compounds are being testing against a variety of enzymes that utilize allylic diphosphates to generate carbocations as a first step of the reaction.
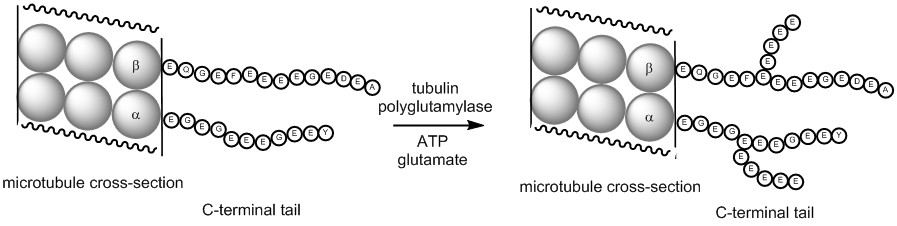
Studies on tubulin posttranslational modifications: Tubulin is an important protein component of the cellular cytoskeleton and plays key roles in a variety of biological processes. Tubulin is extensively post-translationally modified, however the roles of these modifications and the enzymes that catalyze them are poorly understood. The Tanner lab is studying the mechanism and inhibition of the tubulin polyglutamylases (ligases) that add multiple glutamate residues onto the C-terminal tail of tubulin. These ligases may serve as targets for the development of anticancer agents.
- Liu et al., Bioorg. Med. Chem. Lett. 2013, 23, 4408-4412.
Ongoing Research Interests
The Tanner lab has had longstanding research interests in the following areas:
1) Epimerases and racemases. Epimerases and racemases are enzymes that catalyze an inversion of stereochemistry in a biological molecule. Research on both amino acid/peptide and carbohydrate-handling enzymes has been carried out. A particular focus has been placed on enzymes that invert stereogenic centers that do not possess an acidic C-H bond and therefore cannot utilize a simple deprotonation/reportonation mechanism. Enzymes that have been studied include: glutamate racemase, UDP-GlcNAc 2-epimerase, ribulose 5-phosphate 4-epimerase, ADP-heptose 6-epimerase, GDP-fucose synthase.
2) Peptidoglycan biosynthetic/modifying enzymes. Peptidoglycan is the key structural component of the bacterial cell wall and enzymes that target peptidoglycan biosynthesis have long been targets for the development of antibiotics. The Tanner lab has studied enzymes involved in the biosynthesis of peptidoglycan (glutamate racemase, Mur ligases) and in the modification or recycling of peptidoglycan (MurNAc 6-phosphate etherase, O-acetylpeptidoglycan esterase, Csd proteases).
3) Biosynthesis of the sialic acids. Sialic acids are a large and structurally varied family of alpha-keto acids that play key roles in Nature. N-Acetylneuraminic acid is found on the surface of human cells and is a key determinant in the onset of influenza infections. Legionaminic acid is found in the lipopolysaccharide of the causative agent of Legionnaires’ disease. Pseudaminic acid is found as a post-translational modification of the flagellin proteins in the pathogenic bacteria Helicobacter pylori. Enzymes involved in the biosynthesis of these sialic acids have been discovered and studied in the Tanner lab.
4) Enzymes of alkaloid biosynthesis. Alkaloids are a diverse family of nitrogen-containing molecules that often possess potent biological activities. Alkaloids are typically synthesized from amino acids in complex biosynthetic pathways with unusual enzymatic reactions. The Tanner lab has studied the mechanisms of the indole prenyltransferases that are involved in the biosynthesis of prenylated indole alkaloids. One such enzyme is 4-dimethylallyltryptophan synthase that catalyzes an electrophlic aromatic substitution reaction in the first step of ergot alkaloid biosynthesis. Evidence was found for a mechanism involving a C-3 reverse prenylation followed by a Cope rearrangement. Another project involved the enzyme norcoclaurine synthase that catalyzes the first committed step in morphine biosynthesis. This enzyme is an example of a Pictet-Spenglerase and generates a chiral product from achiral starting materials.